CONCLUSIONS
One of the key questions regarding cancer therapies is whether treatment-resistant subclones pre-exist therapy or originate at a later time-point from residual cancer cells that were kept under control until that time.
Here, we addressed this issue in HLA loss leukemia post-transplantation relapses, representing one of the best characterized mechanisms of selective immune escape from the adoptive immune therapy of allogeneic hematopoietic cell transplantation (allo-HCT) ( Vago, NEJM, 2009).
Serial purified acute myeloid leukemia (AML) samples from 11 different patients, collected before allo-HCT and at the time of the post-transplant HLA loss relapse, were subjected to high-depth (average 100x) whole genome sequencing (WGS). Patient and donor germline controls were obtained through in vitro expansion of T cells collected from patients before and after allo-HCT, respectively, and sequenced at an average 30x depth.
Starting from the notion that mutations can be employed as molecular clocks to date the age of cells ( Alexandrov, Nature Genetics, 2015) and that mutational signatures recapitulate their exposure to mutagens ( Alexandrov, Nature, 2020), we combined WGS with Poisson-based modelling to perform subclonal deconvolution ( Caravagna, Bioinformatics, 2020), and derive the timing of the HLA loss rearrangement, intersecting results with the patient clinical history.
The AML cell line OCI-AML3 was employed for validation experiments, assessing in vitro the ability of selected compounds to induce DNA damage (quantifying ϒH2Ax through immunofluorescence and flow cytometry) and specific mutational signatures (by the WGS of single cell clones, performed at an average depth of sequencing of 30x).
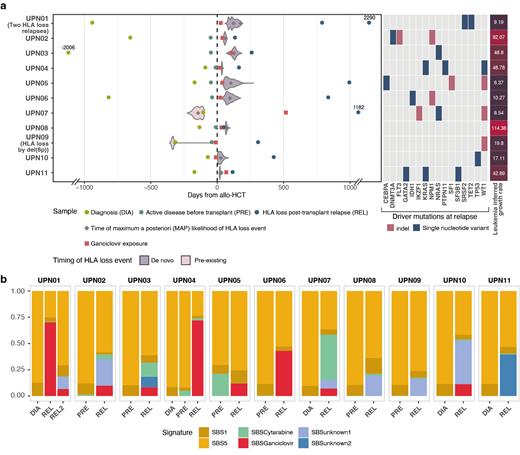
By WGS data of longitudinal AML samples we successfully reconstructed the clonal dynamics leading to 12 HLA loss relapses, and in each subclone deconvolved mutational signatures. By applying Poisson-based timing models to homozygous (i.e. occurred before the copy neutral loss of hetherozygosity - cn-LOH - rearrangement) and heterozygous (i.e. occurred after the event) mutations in the rearranged region, in 10/12 HLA loss relapses we confidently dated the event to a post-transplant time-point (Fig. 1A). In all HLA loss subclones we could detect a C>A mutational signature that was absent in pre-transplant samples (Fig. 1B): combining literature data ( Kanter, Cell Stem Cell, 2021) and in in vitro experiments on the OCI-AML3 cell line, we reconducted this signature to the antiviral drug ganciclovir (GCV). By in vitro experiments, we showed that GCV induces DNA damage and homologous recombination repair in AML, an effect that could be possibly potentiated by the concomitant exposure to the immunosuppressant mycophenolate ( Fang, Genome Medicine, 2022).
Concordantly, by retrospective analysis of 126 AML relapses after a family haploidentical transplantation occurred at our Institute between August 2002 and December 2020, we observed that the incidence of HLA loss was 41/95 (43.1%) in patients exposed to GCV and 5/31 (16.1%) in unexposed ones (OR: 3.94, 95%CI 1.39 to 11.16, p=0.009).
Our data indicate that in the majority of patients HLA loss variants originate after transplant, and that the genotoxicity of ganciclovir is a potent facilitator of the phenomenon. Defining the time of origin of therapy resistance has fundamental clinical consequences, implying that immune escape clones can only be targeted by post-transplant interventions, and warranting the development of new tools to anticipate the rapid detection of relapse.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal